Xanax Recall Due to “Foreign Substance” Presence
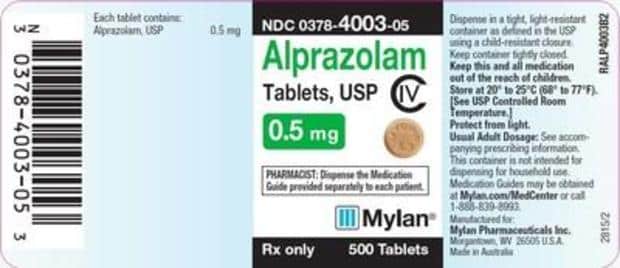
The makers of the popular anti-anxiety drug Xanax is pulling their drug off pharmacy shelves nationwide. Mylan Pharmaceuticals, the makers of Xanax are citing what they call “the potential presence of a foreign substance” for their recall. According to them, there is a “small chance” of infection in the batch of Alprazolam they are recalling. Alprazolam is, of course, the generic name of Xanax. All of this information was posted Saturday by the U.S Food and Drug Administration.
In a statement, Mylan said: “Clinical impact from the foreign material, if present, is expected to be rare, but the remote risk of infection to a patient cannot be ruled out.” The tablets involved are USP C-IV .5mg in 500 count bottles from lot number 8082708. The expiration date of these affected bottles is September 2020. Anyone who filled a Xanax prescription in July through August could have been affected.
If you want to know if you have been affected by this recall, please contact your pharmacy. If they confirm that you received Alprazolam tablets from the affected batch, you can call Mylan to return the product. For instructions on how to return your prescription, call (888) 843-0255 . Consumers with questions about this recall can call Mylan at (800) 796-9526 or email them at customer.service@mylan.com.
Have You Been Harmed From a Defective or Dangerous Drug?
If you have been injured from a dangerous drug? If you have been injured due to a pharmaceutical or medical manufacturer’s negligence, you need an attorney to recover damages for the pain and suffering you have experienced. The Begum Law Group Injury Lawyers knows exactly how to fight these companies to get you the compensation you deserve. Call us today at our office or contact us online. We are available day and night to set up your free consultation.









